Animal studies.
Experiments with various dipeptides containing tyrosine, cystine and glutamine provided compelling evidence that these substrates are readily available and that their constituent amino acids are instantaneously incorporated into the various proteins. tissue.(31-36)
After injecting a bolus or under conditions of continuous total parenteral nutrition (TPN).These peptides supply tyrosine, cystine and glutamine, respectively, to maintain their intra- or extracellular stores.(31-35,37,38)
Parenteral nutrition with dipeptides promotes growth and nitrogen retention .(39-44)
Alanylglutamine administered intravenously reduces glutamine loss in muscles during stress(45) and prevents atrophy of the intestinal mucosa in parenterally fed rats .(46)
Studies in human beings.
Experimental animal studies are perhaps less suitable for investigating amino acid metabolism in cells due to differences in the intracellular distribution of amino acids in human muscle. compared to the animal.(45,47,48)
Therefore, studies in humans are necessary to evaluate the efficacy and safety of short-chain peptides and to determine whether their Intravenous administration favorably influences the overall nitrogen economy in patients or if it entails any other clinical benefit.
Healthy volunteers.
Kinetic studies have shown that alanyl-glutamine,(49) glycyl-tyrosine(49) and alanyl-tyrosine,(50) are rapidly hydrolyzed after being injected as a bolus;The elimination half-life ranges between 3.8 and 5.8 minutes (Table 2).
The apparent volume of distribution was close to 12L, which corresponds to the extracellular compartment.
Table 2. Kinetic values for L-alanyl-L-glutamine (Ala-Gln), glycyl-L-tyrosine (Gly-Tyr), and L-alanyl-L-tyrosine (Ala-Tyr).
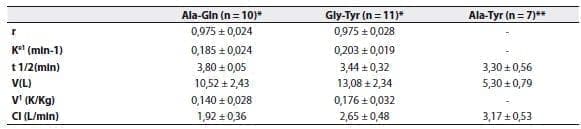
 Values correspond to the mean + SD.r2, determination coefficient;Ke1, elimination rate constant;t1/2, elimination half-life
Values correspond to the mean + SD.r2, determination coefficient;Ke1, elimination rate constant;t1/2, elimination half-life
;V, volume of distribution;V1, distribution coefficient;Cl, plasma clearance.
* Data adapted from Albers et al(49)
** Data adapted from Druml et al(50)
± alt + 0177
I = alt ± -134
Continuous infusion of a commercial amino acid solution supplemented with the two synthetic dipeptides did not produced side effects and did not give rise to complaints.(51)
As a result of the infusion of the solution supplemented with the peptides there was a rapid increase in the plasma concentrations of alanine, glutamine, glycine and tyrosine.
During the entire infusion period, only traces of the dipeptides could be measured in the plasma.Since the values were just above the limit of detection.
Since none of the dipeptides were detected in the urine sample taken at 6 hours, the results pointed towards a “nearly quantitative” hydrolysis. ” of the infused peptides and indicated the subsequent utilization of free amino acids.No appreciable changes in free glutamine concentrations were observed during the infusion of the isonitrogenated control solution, but a continuous decrease in tyrosine levels was observed.
These results have been confirmed by kinetic studies(52,53) and in other investigations in which the efficient use of a solution based on a glycyl dipeptide was demonstrated in healthy volunteers.(54) Loch and his collaborators recently studied the organic elimination of dipeptides containing glutamine in postabsorptive and emaciated humans.(55,56)
Infused peptides are hydrolyzed
These results highlight the fact that infused peptides are easily hydrolyzed in the humans.
Values reflecting an excessively short elimination half-life, exceptionally high peptide clearance rates, and also rapid availability of the constituent free amino acids indicate that the peptides are efficiently assimilated.
The results indicate that alanyl-glutamine, glycyl-tyrosine and other synthetic dipeptides can be used efficiently and safely as a source of free amino acids (glutamine, tyrosine and others). in the context of parenteral nutrition.
Catabolic patients.This obstacle could be overcome by means of synthetic dipeptides containing glutamine.
Infusion for 5 days of a Total Parenteral Nutrition (TPN) solution supplemented with alanyl-glutamine and glycyl-tyrosine in patients undergoing Major elective surgery improved postoperative nitrogen balance, compared to controls that received isonitrogenous and isoenergetic TPN without the peptides(60) (figure 1).
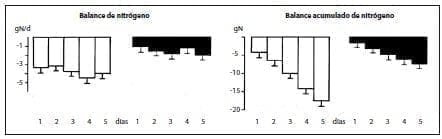

Figure 1.Daily and cumulative nitrogen balance (mean + SEM, n = 6) in patients receiving conventional Total Parenteral Nutrition (TPN) (open columns) or supplemented with alanyl-glutamma (280 mg/kg body weight per day) and glycyltyrosine ( 50 mg/kg body weight per day; black columns), after elective surgery.***p < .00l: **< .01.
This better nitrogen balance was associated with the maintenance of intracellular glutamine stores, which decreased markedly in control patients compared to their preoperative values (Figure 2A).
Muscle tyrosine concentrations decreased when glycyl-tyrosine was not supplemented, while intracellular values improved when glycyl-tyrosine was not supplemented. administered the peptides (Figure 2B).
The peptides could not be detected in either plasma or muscle, and there was no difference in the plasma concentrations of the constituent amino acids between the two treatment groups.
No side effects were observed as a consequence of the infusion of the solutions, and postoperative recovery was normal in all patients.
Intravenous administration of alanyl-glutamine after cholecystectomy contributed to preserving intracellular glutamine stores (91% of the preoperative value) and the characteristic change in the profile of muscle ribosomes was eliminated.(61)
Barua and collaborators reported having obtained beneficial effects with short-term infusion of alanyl-glutamine on protein synthesis in muscles, evaluated based on the incorporation of (13C) leucine in postsurgical patients who were administered parenteral nutrition without glutamine.(62)
In critically ill patients, it was not possible to appreciably influence the intracellular concentration of glutamine in the muscles by administering about 20 g of alanyl-glutamine (13 g of glutamine) daily.(63,64)
However, cumulative nitrogen balance was much better in the group receiving the peptides, and improved on days 1 and 2 (Figure 3).
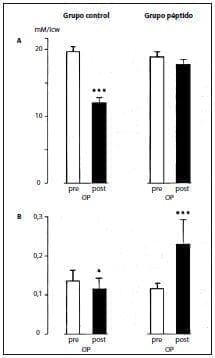
 Figure 2. Concentrations of glutamine (A) and tyrosine (B) in muscle cells (means + SEM, n = 6 in controls and in patients receiving TPN supplemented with alanyl-glutamine (280 mg/kg body weight per day) and glycyl-tyrosine (50 mg/kg body weight per day) , icw, intracellular water;OP, operative.*p <.05;***p < .001.
Figure 2. Concentrations of glutamine (A) and tyrosine (B) in muscle cells (means + SEM, n = 6 in controls and in patients receiving TPN supplemented with alanyl-glutamine (280 mg/kg body weight per day) and glycyl-tyrosine (50 mg/kg body weight per day) , icw, intracellular water;OP, operative.*p <.05;***p < .001.
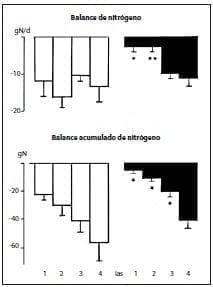
 Figure 3.*p < .05;**p < .01.
Figure 3.*p < .05;**p < .01.
Peterson and their collaborators studied the long-term postoperative effect of Total Parenteral Nutrition (TPN) supplemented with glycyl-glutamine on muscle ribosome profiles and observed that the alterations were less profound than in controls.(65) More importantly, Tremel and colleagues’ observation that glutamine-supplemented parenteral nutrition can prevent the atrophic response of the intestines associated with Total Parenteral Nutrition (TPN) in intensive care patients.(66)
Interpretation of clinical results.
Recent studies carried out in animals have shown that glutamine consumption in the intestinal tract increases notably when there is catabolism.(67-69)
Extrapolating from experimental data, this is approximately 10 to 14 g of glutamine per day in a 70 kg patient.(67)
Including the absorption of about 4 g of glutamine per day by the dog’s kidney(69) and considering the glutamine that rapidly proliferating cells need daily.(70,71) The total glutamine influx could be 14 to 20 g/day.
During stress, the reported value of glutamine released from the muscle varies between 9 and 13 g/day, depending on the severity of the catabolic stimulus.(67,68) It is estimated that glutamine consumption exceeds glutamine release in 5 or 6 g/day.
This figure agrees with the degree of glutamine depletion in the muscle tissue of our surgical patients treated with conventional Total Parenteral Nutrition (TPN) for a period of 3 days.(60)
Thus, by administering about 13 g of glutamine per day in the form of the dipeptide alanyl-glutamine, the depletion of this amino acid in muscle tissue was almost eliminated and the balance was notably improved. of nitrogen.(60)
These results suggest that it is possible to meet the increased cellular demand for metabolic fuel and the intestinal needs of surgical patients through the daily administration of about 13 g of glutamine per day.
On the other hand, the same amount of alanyl-glutamine (20 g/day) did not influence the intracellular stores of free glutamine in cases of severe accidental trauma, although it did improve nitrogen economy in the post-trauma phase.(63,64)
Therefore, the increased intestinal need and cellular demand for metabolic fuel are partially compensated, but not fully satisfied in these patients.
Patients undergoing bone marrow transplant
Wilmore and his collaborators obtained very similar results by supplementing the nutrition of patients undergoing bone marrow transplant with 20 g of glutamine per day bone and whole-body irradiation.(72)
Nitrogen balance improved only during the first few days of the study, after which there was a steady deterioration in retention.
However, when 40 g of glutamine was administered per day, this beneficial effect was prolonged throughout the study.(73)
This blinded controlled study suggests that parenteral nutrition supplemented with glutamine significantly reduces in-hospital morbidity compared to conventional feeding without glutamine.
Contrary to the above, no effect on metabolism and outcome was observed when 20, 40 or 60 g were added. of alanyl-glutamine to the Total Parenteral Nutrition (TPN) of patients with acute pancreatitis.(74-76)
All of these seriously ill patients arrived at the hospital with very low concentrations of intracellular glutamine (2 .8 to 6.6 mmol/L intracellular water).
It would be tempting to speculate whether the ability to utilize substrates is severely limited during critical illness.
It is well known that the utilization of substrates ultimately depends on cell viability and is limited when free energy levels are low.(77)
According to the original hypothesis, the function of intramuscular glutamine was to supply carbon and nitrogen to the liver.And any glutamine deficiency could limit these hepatic processes during catabolic stress.(15,78,79)
Considering what we know today, it would be necessary to review this hypothesis.Glutamine is first of all an essential nutrient for the normal maintenance of the intestinal mucosa( 16) and, supposedly, of proliferating cells.( 70,71)
The supposed increase in the need that Has the gut metabolic fuel during stress could go hand in hand with an increased demand for glutamine in the muscles.
The biggest concern with using free glutamine as Wilmore did in some studies(80,81) is the difficulty of doing it routinely in the clinic.
Due to the instability of free glutamine in aqueous solutions (formation of pyruglutamic acid and ammonia).TPN solutions containing glutamine should be prepared fresh under strict aseptic conditions and stored at 4°C as recommended by Khan and colleagues.(82,83) To reduce the risk of precipitation, the concentration of glutamine in such solutions should not be greater than 1 or 1.5%.
Therefore, administering 40 g of glutamine with this solution to traumatized or critically ill patients is a large burden.Especially if they have fluid restriction.With the help of alanyl-glutamine, highly soluble and stable, it is possible to administer the necessary amount in about 200 mL.
Keeping the solutions at 4 °C can delay the decomposition of glutamine.But there comes a time when they must be heated to make the infusion at a physiological temperature.
The increase in temperature produces a considerable increase in the decomposition products: pyroglutamate and ammonia.It is necessary to define the acceptable limits of these contaminants in intravenous solutions.
There is also a serious risk of bacterial growth in glutamine solutions, particularly when prepared without pharmaceutical advice.
